Today, class started off with Mr. Lieberman telling everyone to get out their Classifying Chemical Reactions Lab so we could go over the post-lab questions, which asked you to find the equations for some experiments that you did during the lab. People wrote the answers on the board and we cleared up any questions about them. For answers see Brandon L's previous post that described the lab's experiments and their equations.
Today, class started off with Mr. Lieberman telling everyone to get out their Classifying Chemical Reactions Lab so we could go over the post-lab questions, which asked you to find the equations for some experiments that you did during the lab. People wrote the answers on the board and we cleared up any questions about them. For answers see Brandon L's previous post that described the lab's experiments and their equations. After we finished discussing the lab, we moved on to the notes for the day. Although we only got through the first part of the notes, we still learned a lot about solutions, and how electrolytes and ions are related. Mr. Lieberman explained that a solution contains a solute, which dissolves, and a solvent, which is what the solute dissolves into. He also explained how water was the most common solvent. As a solvent, water dissociates ionic compounds into its ions. For example if you dissolved table salt (NaCl) into water, then one would see each individual ion separately, because water breaks the Na+ and Cl- apart.
We started to talk about electrolytes, and the difference between strong and weak electrolytes. I general, electrolytes are allowed to pass through a current if there is a sufficient amount of ionization. Strong electrolytes contain enough ions to carry a current efficiently, while weak electrolytes don't completely dissociate and have a small amount of ionization.
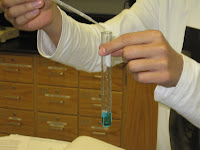
To give us a visual of how electrolytes and ions really worked, Mr. Lieberman performed the light bulb experiment. First he used sugar to see if he could light the light bulb. To fake out the class he screamed to make it seem like there was going to be light. Sure enough, the infamous Korri then proceeded to shriek so loudly, that it was heard around the country, and maybe even the entire world because it was as if every students ear drums were being punctured and then completely ruptured. Mr. Lieberman then demonstrated how the sugar, which has no ions, didn't light the light bulb. He then mixed salt and water together. The water acted as the solvent and the salt was the solute. Na+ and Cl- ions were formed and the light bulb irradiated a lot of light.
You can check out this video, as a woman explains electrolytes and currents.
That's it for class on November 4th.
The next scribe will be the oh so special, John A.