Today and yesterday in Lieb's class we started off by talking about the 3 worksheets we need print off of moodle after this we began our class notes on the 5 types of reactions: Synthesis, Decomposition, Single Replacement, Double Replacement, and Combustion Reactions.
A synthesis Reaction is when you are given two reactants that combine to give you one product for example: 2H2 + 02 -----------> 2H20
A Decomposition Reaction happens when compunds are broken into elements or similar compounds. Example: 2H20 ---------> 2H2 + 02
A Single Replacement Reaction is when an element replaces another compound. These only take place in wate. Example: NaCl + F2 ---------> NaF + Cl2
A Double Replacement Reaction happens when one metal replaces another in a compound and the same for the non-metals. Example: AgN03 + NaCl ----->AgCl + NaN03
A Combustion reacion only occurs when a hydrocarbon reacts with oxygen gas. Example: C5H12 + 802 ------>5CO2 + 6H20
Now on to the Classifying Chemical Reactions Lab YES!!!!!!!!!!!
So, to begin with we went over the Pre-Lab in Question 1 we had to summarize the chemical reaction of sodium bicarbonate, carbon dioxide, and water. This turned into
2NaHC03-----> C02 + H20 + Na2C03. When balanced.
We then had two days to go through the 7 different stations and do all the experiments. In reaction 1 We Burned Magnesium ribbon. The Magnesium ribbon lit on fire and got chalk white after it burned. The chemical Reaction that occured was 2Mg + 02 ----> 2Mg0.


In the first picture we see the product of the Magnesium ribbon turning pure white and in the second it is lighting on fire.
In reaction 2 we put hydrochloric acid in a test tube with Magnesium Metal, and then we lit a wood splint and put it in the tube where it was extinguished. These turned into
2HCl+ Mg ----> MgCl2 + H2

The answer above has been balanced. In this picture we see the hydrochloric acid and Magnesium Metal Ribbon mixing and bubbles occuring.
In reaction 3 we put ammonium carbonate in the test tube an burned for 30 seconds then we put a piece of litmus paper at the top and an odor was released. This odor was amonia next we put a piece of wood flint on fire into the tube which was extinguished. The reaction for this was (NH4)2C03---> C02 + NH3 + H20 when balanced.

In this picture we see the mixture of amonia being released.
In reaction 4 we put calcium carbonate in a test tube with hydrochloric acid and once again lit a wood splint which was extinguished.
This reaction when balance became 2CaC03+ 2HCl-----> 3C02 + 2CaCl + H20.

In the second picture we see the wood splint being extinguished.

In the first picture we see the Calcium Carbonate and hydrochloric acid mixed.
In reaction 5 we mixed copper (II) Chloride and mossy zinc together and the mozzy zinc change color from silver to read. CuCl2 + Zn-------> ZnCl2 + Cu. When balanced.
In this picture we see the zinc changing color.

In reaction 6 we added copper chloride with sodium phosphate and a precipitate formed. When this reaction was balanced it became
3CuCl2 + 2Na3P04------> Cu3(PO4)2 + 6NaCl
In this photo you can see the precipitate forming.
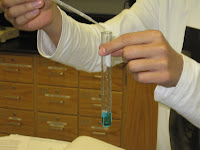
In reaction 7 we mixed sodium hydroxide and phenolphthalein and the hydrochloric acid. During this reaction it at first became pink and then back to clear liquid color whise. In this reaction when balanced it became NaOH + HCl-----> H20 + NaCl As you can see in the pictures below it demonstrates the pink on the right and then clear again on the left


Well that was our class in a nut-shell and the coveted spot of scribing for Thursday goes to my man Josh D CONGRATS!!!
No comments:
Post a Comment