Today was a notes day. We explored how certain conditions, specifically temperature and pressure, impacted the solubility of solutions. Each of these conditions affects their solute differently based on its state of matter; a solid, gas, or liquid.
An increase in temperature for solids and liquids results in an increase of solubility, as the intensified intermolecular motion allows for a more thorough interaction between the solute and solvent. However, higher temperatures in gases results in molecules being released to the atmosphere instead of contained within the solvent, resulting in lower solubility.
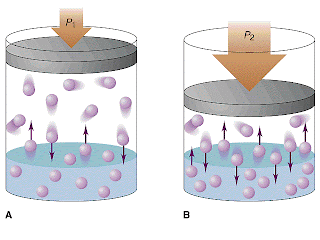
Pressure only changes the solubility of gases. Solids and liquids already have their molecules tightly packed so that an increase in pressure would not change the structure of the solute enough to change solubility. Gases are more prone to be altered by pressure because their molecules are more spread out. The increase in pressure forces the molecules of gas down into the solvent, increasing solubility.
We discussed real world examples describing the relationship between pressure and solubility. Soda is kept under high pressure in its can. When that pressure is released when the can is opened, pressure no longer forces the CO2 molecules in to the soda. The CO2 rises to the top in the form of bubbles and thus, carbonation.
Here's some scientific photos to help explain:
BEFORE:

AFTER:

It seems as though a rapid decrease in solubility also has the side-effect of a spontaneous age, gender, and race change.
I guess the next scribe is Michelle T., to fill her duties from today.
No comments:
Post a Comment