2C2H2 + 5O2-> 4CO2 + 2H2O
From this Mr. L asked us how many grams of H2O will form if .60 grams of CAC2 reacts. This is the formula we got:
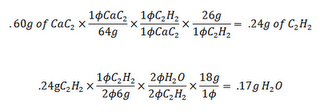
We ended up with .17 grams of water. Now it was time to put this formula to the test. Mr. Lieberman loaded up his mole-rocket with the special ingredients from the formula. It was the combination of the CaC2 and H2O that created the Acetylene gas. This gas was the ignition that the rocket needed to make the mole fly. Suddenly there was a BOOM! A little red object shot across the room followed closely by a trail of fire. There were a few screams, particularly from Korri, which was expected. The room then filled with a smell that was immediately placeable: It was a mix of burnt hair and/or that smell from straightening your hair when it is still slightly damp.
Next, we all took our 4 question quiz. It was then graded and handed back. Hope everyone did well! And the next scribe issssss........Mollie M!
No comments:
Post a Comment