
In class today, we started a lab with many different sodium and nitrate groups, totaling 48 reactions. Why would we do that many, you ask? Well it has to be either:
a) Mr. Lieberman likes to make us do a ridiculous amount of writing and balancing equations
b) To help us formulate rules on formation of precipitates.
or c) All of the above
I'm Gonna go with C.
To start off, we put various solutions containing water and a Sodium Ion in each column. Then, we added a Nitrate solution to each of those, receiving varying results:
Some did absolutely nothing, leaving a clear liquid behind.
Others changed colors, but no precipitate formed.
Still others formed either a cloudy or completely solid precipitate, meaning that a reaction occurred.
And a couple did this:
Just kidding. But still, it was exciting. Here are the real results:
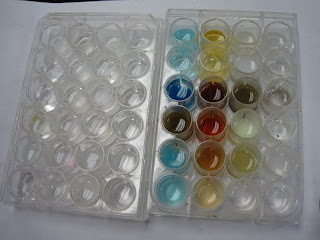 And my data table, if you can read it:
And my data table, if you can read it: And the post lab is the real fun part: we have to write chemical equations for EVERY SINGLE PRECIPITATE! Both molecular and net ionic. For example, the molecular equation would be: 3Na2SO3+2Al(NO3)3 yields Al2(CO3)3+6NaNO3 and the net ionic equation would be: 2Al3++ 3CO32 yields Al2(CO3)3. And repeat. About 20 times. Just a thrilling homework assignment. Oh, and by the way, for those who can't realize it yet: NaNO3 is a product for every single molecular reaction, and always dissolves.
And the post lab is the real fun part: we have to write chemical equations for EVERY SINGLE PRECIPITATE! Both molecular and net ionic. For example, the molecular equation would be: 3Na2SO3+2Al(NO3)3 yields Al2(CO3)3+6NaNO3 and the net ionic equation would be: 2Al3++ 3CO32 yields Al2(CO3)3. And repeat. About 20 times. Just a thrilling homework assignment. Oh, and by the way, for those who can't realize it yet: NaNO3 is a product for every single molecular reaction, and always dissolves.Thank you for reading my post. I hope you all do wonderful on your labs now. Now, the next scribe will be:.............................................
Ben A.
No comments:
Post a Comment