A peek inside the everyday happenings of our classroom. This is an interactive learning environment for students and parents in my Honors Chemistry 173 class. This ongoing dialogue is as rich as YOU make it. Visit often and post your comments freely.
Wednesday, February 2, 2011
Entropy and Gibbs free energy
GIBBS FREE ENERGY
Defined: the energy in the system that is available to do useful work.
It is given this symbol and can be calculated like this
Remember that Gibbs free energy is measured in Kj/Mol
THINGS YOU SHOULD KNOW
- If
is negative the forward reaction is spontaneous
- If
is negative then A+b ------> C would be spontaneous
Δ G Reaction Behavior Negative Proceeds spontaneously to the right Zero Is at equilibrium Positive Will not proceed
Below is the entropy post
ENTROPY SHENANIGANS
We started off class today with lab review I am going to type on of the reactions that we reviewed and if you have any questions about the second two reactions, post a comment and I, or someone else, will do their best to answer.
REACTION ONE
Mg + 2HCl ------> MgCl2 + H2
You can use q=mc
BUT WAIT HOW DO YOU GET THE MASS!!!!
Though only the people who got the Mg would know you use a simple conversion factor of
After that you would find the moles of magnesium by these equation
REMEMBER – this was done twice – you need to do the process above two times and then calculate the average. THIS GOES FOR ALL THREE REACTIONS DON’T FORGET THIS THE MORE ACCURATE YOU ARE THE LOWER YOUR PERCENT ERROR WILL BE
Once you have all three of the equations done you need to use Hess’s law AND SHOW YOUR WORK. This work should be shown in the evidence section that lap
CHEM NOTES
Seeing As the slide share link always takes me to gun manuals I am going to type out my notes.
- We started with spontaneous reactions
- A SPONTANEOUS REACTION IS A REACTION THAT TAKES PLACE ON ITS OWN WITHOUT OUTSIDE FORCES
- there are a few things to be worried about though, LIKE
i. It does not have to start on its own so long as it carries out the rest of the experiment
ii. If it spontaneous in one direction then it is not spontaneous in the other direction
- Examples of spontaneous reactions
- Hydrogen ballon reaction
- Paint can explosion
- Ice cube melting
- RUBBER BAND
i. This was the one we spent the most time on
1. The reason that it is such a good example was because when a rubber band is left to sit, it is going to sit there and not move. No matter how much you want something to happen without touching the rubber band, it wont happen. However if stretched out and then contracted, that contraction is a spontaneous reaction because it goes from being stretched out to a normal state with no outside influence
ii. You should not that spontaneous reactions have nothing to do with speed. Below is a great picture for the visual learners out there. if it doesn't show up the it is also here
iii. 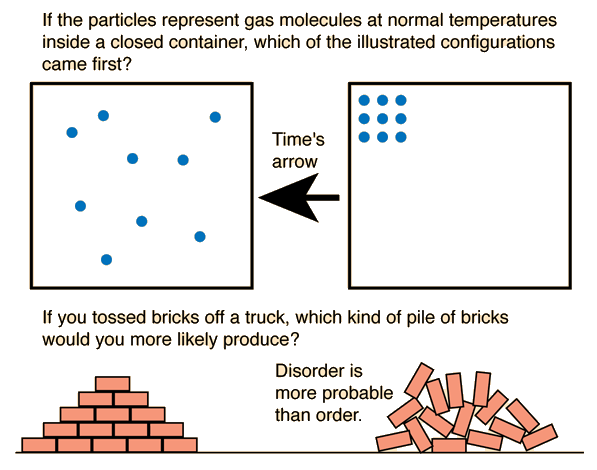
- Nature allows spontaneous reactions
i. Nature divides spontaneous reactions in two main ways
1. Maximum probability – this is where everything mixes
2. Minimum probability – nature has the chance to become disordered but does not
- ENTROPY
- Entropy is denoted with “S”
- Defined as – the increase in disorder or randomness
- REMEMBER TO USE j/mol K NOT Kj/mol K
i. Nature always wants to move toward a positive entropy
- MICROSTATES: different ways molecules can be distributed
i. Increase of microstates = an increase of entropy
ii. large number of microstates = large probability of different states = higher entropy
- FACTORS THAT INFLUENCE ENTROPY
i. Liquid has a great entropy than solid
ii. Gas has the highest
- CALCULATING ENTROPY
i. Very similar to calculating enthalpy
1. It is
2. REMEMBER COEFFICIENTS ARE USED IN THE EXACT SAME WAY WHEN CALCULATING ENTROPY AS THEY ARE WHEN YOU ARE CALCULATING ENTHALPY
a. If there are 2mol of something you multiple by 2 etc
http://www.youtube.com/watch?v=B4SFv_2Skdc&feature=related
This video does a good job summarizing the lesson today.
Alright well I am about done there are just a few announcements you should be aware of
- Today is Kathryn J’s birthday if you haven’t wished her a happy birthday DO IT
- Mr. Lieberman has his ear pierced, though he never wears it
- The lab is due tomorrow
- There is a web assign due Wednesday
- The class would like to congratulate Mollie and Emilio for… Well, being Mollie and Emilio ;)
- We got a Worksheet today that you should do
- Everything for this chapter including both sets of book problems will be due on Thursday and if we have a snow day on Wednesday well then I am not sure.
That’s about it for today. The next scribe is Kathryn J, enjoy
Tuesday, February 1, 2011
units for entropy
Monday, January 31, 2011
Entropy
REACTION ONE
Mg + 2HCl ------> MgCl2 + H2
You can use q=mc
BUT WAIT HOW DO YOU GET THE MASS!!!!
Though only the people who got the Mg would know you use a simple conversion factor of
After that you would find the moles of magnesium by these equation
REMEMBER – this was done twice – you need to do the process above two times and then calculate the average. THIS GOES FOR ALL THREE REACTIONS DON’T FORGET THIS THE MORE ACCURATE YOU ARE THE LOWER YOUR PERCENT ERROR WILL BE
Once you have all three of the equations done you need to use Hess’s law AND SHOW YOUR WORK. This work should be shown in the evidence section that lap
CHEM NOTES
Seeing As the slide share link always takes me to gun manuals I am going to type out my notes.
1. We started with spontaneous reactions
2. A SPONTANEOUS REACTION IS A REACTION THAT TAKES PLACE ON ITS OWN WITHOUT OUTSIDE FORCES
a. there are a few things to be worried about though, LIKE
i. It does not have to start on its own so long as it carries out the rest of the experiment
ii. If it spontaneous in one direction then it is not spontaneous in the other direction
3. Examples of spontaneous reactions
a. Hydrogen ballon reaction
b. Paint can explosion
c. Ice cube melting
d. RUBBER BAND
i. This was the one we spent the most time on
1. The reason that it is such a good example was because when a rubber band is left to sit, it is going to sit there and not move. No matter how much you want something to happen without touching the rubber band, it wont happen. However if stretched out and then contracted, that contraction is a spontaneous reaction because it goes from being stretched out to a normal state with no outside influence
ii. You should not that spontaneous reactions have nothing to do with speed.
iii.
e. Nature allows spontaneous reactions
i. Nature divides spontaneous reactions in two main ways
1. Maximum probability – this is where everything mixes
2. Minimum probability – nature has the chance to become disordered but does not
4. ENTROPY
a. Entropy is denoted with “S”
b. Defined as – the increase in disorder or randomness
i. Nature always wants to move toward a positive entropy
c. MICROSTATES: different ways molecules can be distributed
i. Increase of microstates = an increase of entropy
ii. large number of microstates = large probability of different states = higher entropy
d. FACTORS THAT INFLUENCE ENTROPY
i. Liquid has a great entropy than solid
ii. Gas has the highest
e. CALCULATING ENTROPY
i. Very similar to calculating enthalpy
1. It is
2. REMEMBER COEFFICIENTS ARE USED IN THE EXACT SAME WAY WHEN CALCULATING ENTROPY AS THEY ARE WHEN YOU ARE CALCULATING ENTHALPY
a. If there are 2mol of something you multiple by 2 etc
d
This video does a good job summarizing the lesson today.
Alright well I am about done there are just a few announcements you should be aware of
1. Today is Kathryn J’s birthday if you haven’t wished her a happy birthday DO IT
2. Mr. Lieberman has his ear pierced, though he never wears it
3. The lab is due tomorrow
4. There is a web assign due Wednesday
5. The class would like to congratulate Mollie and Emilio for… Well, being Mollie and Emilio ;)
6. We got a Worksheet today that you should do
7. Everything for this chapter including both sets of book problems will be due on Thursday and if we have a snow day on Wednesday well then I am not sure.
That’s about it for today. The next scribe is Ben T, enjoy
Sunday, January 30, 2011
Hess's Law Lab
First, we made our date tables, which should ideally include two separate areas (for Equation A and Reaction #2) with spaces for two trials. We began Equation A by measureing two strips of magnesium ribbon and recording that in our data table. We then massed a clean dry calorimeter, and remassed it once we added 15 mL of HCl (recording both in the data table, of course). We took the initial temperature of the HCl, and then added a piece of magnesium to the calorimeter. We stirred the piece as it dissolved, and then retook the temperature once it was constant. Once that was over and we recorded all of those temperatures and masses in our data table, we repeated!
Then, it was on to Reaction #2. We remassed the calorimeter (once it was clean and dry), and remassed once we added 15 mL of HCl. To switch things up, we then added about .20 g of magnesium oxide. We recorded the masses of the calorimeter, as well as the actual mass of the magnesium oxide in our data table. Once we added the magnesium oxide in the calorimeter, we stirred until the temperature remained constant. We recorded this temperature and then repeated the whole process!
Once this lab was over, we left chemistry to go enjoy our weekends. Don't forget to work on this lab, though, it's due Tuesday! Good luck to Bobby S, our next scribe, on his first post for Period 6!