To make things more interesting, Mr. Lieberman did the Lead Iodide experiment to show that certain compounds are always soluble (NO3), and therefore, you are able to identify the solids in a reaction. See Video Below:
A peek inside the everyday happenings of our classroom. This is an interactive learning environment for students and parents in my Honors Chemistry 173 class. This ongoing dialogue is as rich as YOU make it. Visit often and post your comments freely.
Tuesday, November 9, 2010
Insolubility and Solubility
To make things more interesting, Mr. Lieberman did the Lead Iodide experiment to show that certain compounds are always soluble (NO3), and therefore, you are able to identify the solids in a reaction. See Video Below:
Monday, November 8, 2010
Lots of Reactions

In class today, we started a lab with many different sodium and nitrate groups, totaling 48 reactions. Why would we do that many, you ask? Well it has to be either:
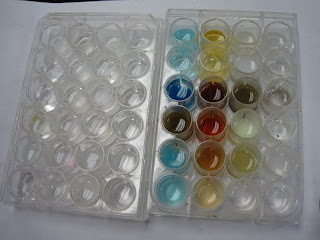 And my data table, if you can read it:
And my data table, if you can read it: And the post lab is the real fun part: we have to write chemical equations for EVERY SINGLE PRECIPITATE! Both molecular and net ionic. For example, the molecular equation would be: 3Na2SO3+2Al(NO3)3 yields Al2(CO3)3+6NaNO3 and the net ionic equation would be: 2Al3++ 3CO32 yields Al2(CO3)3. And repeat. About 20 times. Just a thrilling homework assignment. Oh, and by the way, for those who can't realize it yet: NaNO3 is a product for every single molecular reaction, and always dissolves.
And the post lab is the real fun part: we have to write chemical equations for EVERY SINGLE PRECIPITATE! Both molecular and net ionic. For example, the molecular equation would be: 3Na2SO3+2Al(NO3)3 yields Al2(CO3)3+6NaNO3 and the net ionic equation would be: 2Al3++ 3CO32 yields Al2(CO3)3. And repeat. About 20 times. Just a thrilling homework assignment. Oh, and by the way, for those who can't realize it yet: NaNO3 is a product for every single molecular reaction, and always dissolves.Thank you for reading my post. I hope you all do wonderful on your labs now. Now, the next scribe will be:.............................................
Sunday, November 7, 2010
Keeping Balancing Equations Cool Like They're Going Out of Style
- Molecular Equations show the overall stoichiometry of the equation, but not the actual forms of the substances
- (Example): KCl+AgNO3------------>KNO3+AgCl
Complete Ionic Equations
- Complete Ionic Equations represent, as ions, all substances that are strong electrolytes
- Ionic Compounds dissociate in water
- Spectator Ions are ions that don't react; spectator ions are still included in Complete Ionic Equations
- (Example): K(^+)+ Cl(^-)+NO3(^-)------------>AgCl+K(^+)+NO3(^-)
Net Ionic Equations
- Includes only those ions undergoing a charge (spectator ions are NOT included)
- Net Ionic Equations are only written if a solid is formed
- (Example): Cl(^-)+Ag(^+)------------->AgCl(s)
NOTE: Molecular Equations, Complete Ionic Equations, and Net Ionic Equations are all Double Replacement Reactions
After we deciphered Molecular Equations, Complete Ionic Equations, and Net Ionic Equations, we proceeded to work on our newest worksheet (Net Ionic Equation Worksheet). Here are some tips in completing this worksheet:
- To find the Molecular Equation, balance the equation given
- To find the Complete Ionic Equation, seperate ions from compounds (include charges)
- If a reaction isn't seen, or there is no solid, a Net Ionic Equation isn't needed
We proceeded to work on the Net Ionic Equation Worksheet for the rest of class.
On Monday, we will be doing a series of 48 reactions. The reactions will help establish rules regarding what we talked about on Friday. Furthermore, there is a WebAssign due Monday, on section 4.2 (Precipitation Reactions).
Stay thursty, my friends.