
A peek inside the everyday happenings of our classroom. This is an interactive learning environment for students and parents in my Honors Chemistry 173 class. This ongoing dialogue is as rich as YOU make it. Visit often and post your comments freely.
Sunday, April 10, 2011
I hate life

Wednesday, April 6, 2011
The Ninja From Down Under


 Today we had shortened classes and Mr. Liberman was not there. So, we had a sub and we got to watch Myth Busters! SWEAAAAAAAAAAAAT! This episode was the second one they have done on ninjas. They were retesting if a ninja could actually catch an arrow, but this time they had the most accomplished ninja in the world today who happened to be Australian. Adam and Jamie the two hosts talked about how that the speed of an arrow does not change on release from the bow to impact with its target. The first experiment was to see if the ninja could catch tennis balls going 80 mph's and he caught 22 in a row a Guiness World Record. Then Jamie tried to accomplish this feat thinking it was being overplayed he was soon pelted relentlessly with thennis balls quite amusing. Next, Carry, Grant, and Tory tested the old assination technic that ninjas would lay in wait in river banks breathing out their blow guns and then using them to kill their target. First they checked how long Tory could stay underwater with out suffering hypothermia and he felt he could last about an hour. Next they tested if they used an all natural blow gun how their aim would be. Tory and Grant made all natural ones but they accuracy was pitiful. Next, Carry who cheated by putting a metal rod in had perfect accuracy. Next she sat underwater and had to deal with the refraction of the water to hit the target. Eventually she go the hang of it. Next they tried to put all the aspects together breathing through it, being cold, and having to transfer the dart in. The myth was busted because no one could load the dart without shooting it with water. Back to Jamie and Adam they put their ninja to the test when he caught an arrow then they put him between three archers and at first he could not do it. Eventually when they moved back and the one who had to shoot had to draw his bow the ninja caught it. The next segment was supposed to be the one inch punch but we did not get to it. Thats it the next scrib is Matt Park
Today we had shortened classes and Mr. Liberman was not there. So, we had a sub and we got to watch Myth Busters! SWEAAAAAAAAAAAAT! This episode was the second one they have done on ninjas. They were retesting if a ninja could actually catch an arrow, but this time they had the most accomplished ninja in the world today who happened to be Australian. Adam and Jamie the two hosts talked about how that the speed of an arrow does not change on release from the bow to impact with its target. The first experiment was to see if the ninja could catch tennis balls going 80 mph's and he caught 22 in a row a Guiness World Record. Then Jamie tried to accomplish this feat thinking it was being overplayed he was soon pelted relentlessly with thennis balls quite amusing. Next, Carry, Grant, and Tory tested the old assination technic that ninjas would lay in wait in river banks breathing out their blow guns and then using them to kill their target. First they checked how long Tory could stay underwater with out suffering hypothermia and he felt he could last about an hour. Next they tested if they used an all natural blow gun how their aim would be. Tory and Grant made all natural ones but they accuracy was pitiful. Next, Carry who cheated by putting a metal rod in had perfect accuracy. Next she sat underwater and had to deal with the refraction of the water to hit the target. Eventually she go the hang of it. Next they tried to put all the aspects together breathing through it, being cold, and having to transfer the dart in. The myth was busted because no one could load the dart without shooting it with water. Back to Jamie and Adam they put their ninja to the test when he caught an arrow then they put him between three archers and at first he could not do it. Eventually when they moved back and the one who had to shoot had to draw his bow the ninja caught it. The next segment was supposed to be the one inch punch but we did not get to it. Thats it the next scrib is Matt Park
Tuesday, April 5, 2011
The Slimy Blue But Also Clear Stuff Lab
Colliding into Activation Energy
 The Kinetic energy required to break the bonds in a molecule and cause a chemical reaction is known as the Activation Energy. It is also referred to as the Activated Complex. It is at the top of the graph showing a reaction's energy, just like this one:
The Kinetic energy required to break the bonds in a molecule and cause a chemical reaction is known as the Activation Energy. It is also referred to as the Activated Complex. It is at the top of the graph showing a reaction's energy, just like this one:  We only discussed one way to increase the speed of this type of reaction, although there are many ways to do this. The one we discussed is by adding a catalyst that lowers the activation energy which allows a higher number of reactions to occur among the molecules in the reaction. The catalyst is not a part of the reaction, though, so it will not appear in the reactants or products. The next Scribe will beeeeee: Kaitlin S. Goodluck
We only discussed one way to increase the speed of this type of reaction, although there are many ways to do this. The one we discussed is by adding a catalyst that lowers the activation energy which allows a higher number of reactions to occur among the molecules in the reaction. The catalyst is not a part of the reaction, though, so it will not appear in the reactants or products. The next Scribe will beeeeee: Kaitlin S. Goodluck
Wednesday, March 23, 2011
Colligative Properties Lab
Today in Chemistry we did the Colligative Properties Lab. The goal of this lab was to use boiling point elevation data to identify an unknown salt. We began by labeling four 100 mL beakers A,B,C, and D and recording their masses in our super neat data tables. Next we filled each of the beakers about half full with distilled water. We recorded the mass of the beaker and water in our data table. We then placed the beakers full of water on the hot plate and heated them to about 85°C at which point we removed them from the hot plate. We determined the boiling point of the water in beaker A by noting the plateau on our lab pro. We recorded this number and proceeded to add about 5.0 grams of the unknown ionic solid to beaker B. We placed it on the hot plate and recorded its boiling point in our data table. We then added about 10.0 grams of the unknown solid to beaker C. We placed it on the hot plate and recorded its boiling point once again. Finally we added 15.0 grams of the unknown solid to beaker D, placed it on the hot plate, and recorded its boiling point in our data tables.
As a part of the data/calculations we were asked to calculate the molality of each solution and were given the molal boiling point elevation constant: 0.51°C kg/mol. To calculate the molality of each solution, we used the equation ΔTb = Kb · m · i. To calculate change in temperature, we subtracted the boiling point of beaker A from beaker B, beaker B from beaker C and so on. We were told the ionic sold has 2 ions, so i=2. We plugged these values into the equation and solved for m to get a value for molality.
To calculate the moles of solute in beakers B, C, and D, we used the molality equation which says that molality = mol solute / kg solvent. We have the value for molality and to find the kg of solvent we simply subtracted the mass of the beaker from the mass of the beaker and water and divided the answer by 1000 to obtain the value in kilograms. To find the moles of solute, we multiplied the molality by kg solvent.
To find the molar mass of the solute in beakers B,C, and D we divided the number of grams of solute in each mixture by the moles of solute obtained in the previous step. We added 5.0 grams to beaker B, 10.0 grams to beaker C, and 15.0 grams to beaker D. These values were divided by the moles solute to obtain the molar mass of the solutes. To calculate the average of the molar masses, we just added them together and divided by three.
The conclusion asks you to decide the formula for the unknown solute, so choose the formula with the molar mass closest to your average. The choices are NaCl, KI, NaNO3 or NaBr. Support your claim with evidence and then calculate the percent error:
| actual value – theoretical value | x 100 %
theoretical value
The lab is due Friday and so are all of the worksheets and Webassigns. Study for the Test Friday!
The next scribe will be Emilio I!
Tuesday, March 22, 2011
Title
Monday, March 21, 2011
Stoichiometry Expanded
When then preceded to solve this problem for practice:
How much calcium carbonate will be precipitated by adding 25.0 ml of calcium chloride to 25.0ml of 56 M potassium?


Remember to keep doing those web assigns and worksheets for preparation for the test on friday!
The next scribe will be Ben T !
Saturday, March 19, 2011
Molarity vs Molality vs Molasses
We then continued to solve some example problems of calculating concentrations. Take a look at your notes for some extra practice.
Molarity and Dilution
At the end we introduced the concept of Molarity and dilution, and established this formula
m1v1 = m2v2.
Here is a video walking through the conversation of Molality to Molarity.
Here doesn't sound very happy, but he is pretty concise. Also remember to do your webassigns and homework. Also molasses has no link to chemistry if you were curious,but they did come to mind.
Thursday, March 17, 2011
Heat, Cool and Repeat
The rest of class was working on our new Solubility Curve lab (due Monday).
This took up the whole classtime and we even had to split up the work amongst our groups to get the job done faster. Essentially, we had two "series" to run, each with the same procedures but with different masses for the materials in the three test tubes.
In the first session, test tube A had to contain 0.45 to 0.50 grams of potassium nitrate (KNO3), test tube B had 0.70 to 0.80 grams, and C had to have 1.20 to 1.30 grams.
I massed each of these tubes and then with the KNO3. Then I massed each test tube with water added. From there, I placed each test tube into boiling water and stirred them gently (well actually, prodded at them more) to make the KNO3 dissolve faster. Once it was completely dissolved, I first took out test tube C and placed it in a beaker of iced water to make it crystallize. The temperature at which test tube C started turning white was recorded. This is the same as the saturation temperature. What was done to test tube C was then done for the other test tubes.
The second series (that was being tested at the same time) had test tube A massed at 0.35 to 0.40 grams, B with 0.90 to 1.00 grams and C with 1.50 to 1.60 grams. My partners then repeated the process I explained above.

<------(crystallized KNO3)
There are some calculations and a graph to do on the computer to go with this lab, along with the conclusion.
(There is also a Webassign due at 1:00 Friday.. just in case)
The next scribe will be.....Austin W.
Wednesday, March 16, 2011
Factors Affecting Solubility



Tuesday, March 15, 2011
Solution Formation Lab

For our groups procedures, we kept all of the variables the same except for the one we were testing, in order to make sure the that change in results is because of the change on the variable that we controlled. For example, the first part of the lab was to determine how the size of the crystal effected the solution formation. By testing three different sized crystals of copper sulfate at the same temperature, our group got the conclusion that the larger the crystal, the longer it takes for it to dissolve.
So, that is pretty much it for our shortened class.
The next scribe will be Michelle T.
Monday, March 14, 2011
Let Chemistry Absorb You. Ha.
- A solute is the substance that is being dissolved
- A solvent is the liquid in which the solute is dissolved
- Solute dissolves in solvent
- Aqueous = solution with water as solvent
- Primarily, there is the separation of a solute, and in order of this to happen, the solute's molecules must surpass their intermolecular forces (IMF) and it requires energy, making it endothermic.
- Secondly, the separation of a solvent occurs when the solvent overcomes its intermolecular forces. This also requires energy, also making it endothermic.
- Thirdly, the interaction of these two substances occurs. An attractive bond forms between the solvent and solute molecules and this releases energy, making it exothermic.
Wednesday, March 9, 2011
Slam a revolving door
Besides from the obvious... today in class, first thing we did was review the forces. We reviewed the dispersion forces, dipole forces, and hydrogen bonding. for more information you can look below at Elim's post.
After our review, we started our lab.
This lab has 7 stations to observe trends and patterns in several properties of liquids and to use a model of intermolecular forces to explain the findings.
Station 1: Evaporation Rates
This station measured the rate at which the liquid evaporated. the rate that the liquid evaporates depends on the strength of the intermolecular forces between liquid molecules. liquids with the lowest evaporation rates have the strongest intermolecular forces.
Insert Photo here that I could not find
 Station 2: Capillary Action
Station 2: Capillary ActionA liquid tends to climb up the walls of narrow columns, this is known as capillary action. This is based upon two forces: adhesive forces, and cohesive forces. The greater the cohesive strength, the more the liquid climbs the column. The greater the adhesive strength, the lesser the liquid climbs the column.

Station 3: Vortex Formation and Relaxation
If you swirl water, you get a water tornado. That's what this is, just making vortex's by swirling the liquids. The time it takes for a liquid to settle down from a swirl is different for all liquids depending on the intermolecular forces. the longer it takes to relax, the stronger the intermolecular forces are. We did this with three liquids: Water, Hexane, and Heavy oil.
Station 4: Viscosity or Resistance to Flow
Not every liquid flows at the same rate (compare water to waffle syrup). Viscosity is the property of a liquid which provides a measure of that liquids tendency to resist flow. Liquids with stronger intermolecular forces have a greater resistance to flow, a higher viscosity. We took the same three substances as station 3, and put them in test tubes. After that we timed the seconds of a marble falling to the bottom of the test tube. The faster it is, the lower the liquids tendency to resist flow, or the lower the Viscosity. The lower the viscosity, the weaker the intermolecular forces.
Station 5: Surface Tension
Molecules on the very surface of a liquid can form strong enough intermolecular forces between them to make their surface impenetrable. It's this surface tension (along with other factors) that make leaves floar on water. For this expirement, we had to lower a paperclip using a tray to try to make it float on the liquid. The liquid that the paperclip can most easily float has the greatest surface tension.

Station 6: Beading
Many liquids have a tendency to bead. For example water droplets that look circular, that is actually the water beading. This is cause by liquid molecules to form a strong intermolecular attraction with itself and curl into a spherical bead or droplet. The greater the strength of the intermolecular forces, the greater the tendency of the liquid to bead. If placed on a surface the liquid is attracted to, the liquids beads less. For this expirement we dropped droplets of liquids onto wax paper and our lab benches. We then made observations according to what beaded and what didnt.
Station 7: Freezing point and Boiling Point
unlike the other stations, this one was all on our packet. all liquids have a freezing and boiling point. These points depend on the strength of the intermolecular forces. Liquids with stronger intermolecular forces have higher boiling points. The graph on our paper is pretty straightforward if you just remember that.
Don't forget we have a ton of webassigns due on Friday as well as this lab.
This lab must also have a conclusion on a seperate piece of paper (though the data can all be in the packet).
The next scribe will be Emilio.
Tuesday, March 8, 2011
Polar and Non-Polar molecule
Mr. Liberman said most of us didn't understand about "stable."
When atoms are stable that means they don't want to bond or already bonded.
And he said we can't break octet rule except inside. In other words, outside of elctrons can't have electrons over 8.
After that he explaind about polar molecule and non-polar molecule by showing us a great demonstration.
He had two buret, a vertical cylindrical piece of laboratory glassware, that one has filled up with water and the other one has filled up with acetone.
By rubbing his hair with a balloon, that balloon has negatively charged.
As he moved the balloon close to the buret which has water, the flow of the water started to bend toward the balloon. It happens because water is positively charged and it is polar molecule.
Theoretically, acetone has to be not bend but it did. Mr. Liberman guessed the acetone mixed with water.
Anyway acetone can not be bend because it is negatively charged and non-polar molecule.
+ Non-Polar molecule
-charge is evenly spread out in the molecule
-NO NON-BONDING PAIRS
+ Polar molecule
-HAS NON-BONDING PAIRS
-the negatively charged center atom balances the molecule with positively charged outside of atoms
The main difference between non-polar and polar molecule is that non-polar molecule does not have non-bonding pairs and polar molecule has non-bonding pairs.
To determine polarity you should look for non bonding pair.
Mr. Liberman also explained different kind of forces-intermolecular, dispersion, and dipole forces.
Every molecules have intermolecular and dispersion forces.
Dispersion force is not that strong force but attractive.
Bigger molecule has lots of dispersion forces.
When he sprinkled acetone on his hand it disappeared very quickly but water did not.
This is a demonstration of dispersion forces.
Also we learned about hydrogen bonding which is strongest force.
I am really sorry that I couldn't explain very well..
So if you need more information about this stuff, I recommend you to visit here:
http://www.tutorvista.com/chemistry/difference-between-polar-and-nonpolar-molecules
And just a reminder, we have a lot of webassign to do.
Also we have a test on Friday.
The next scribe will be Alex K.
Sunday, March 6, 2011
Sharing is Caring
First, we learned about polar and non-polar. These are only between 2 nonmetals.
Polar covalent bonds are where electrons are not shared equally between molecules, and the electronegativity difference is between 0.4 and 1.7.
Non-polar covalent bonds are where electrons are shared equally between molecules, and the electronegativity different is between 0 and 0.4.
An example of a non-polar covalent bond is C-C. It is said to be like "tug of war with your twin". No one would win and the forces trying to win, or gain the electrons are equal, so the electrons would be shared equally. The electronegativity for the two atoms are the same, so they are equally pulling for the electrons. This is the scenario where Mr. Lieberman gave half his candy to Zoe and kept half for himself.
An example for a polar covalent bond would be C-F. The electronegativity for fluorine is strong than that of carbon, and therefore, there is an unequal share of electrons, because flourine would be winning the game of tug of war with carbon. Fluorine becomes more negative because the electrons are closer to fluorine than carbon, even though they are still shared with carbon, so that would mean fluorine is partially negative. Carbon has less of a pulling force on the electrons so it is partially positive. The opposite ends (+ and -) create a dipole. This is the scenario where Mr. Lieberman gave one piece of candy to Zoe and ate the rest.
Ionic bonds are where one molecule gets all the electrons, having a complete transfer, even though this is considered sharing. The electronegativity of one nonmetal atom is so much greater than the other metal atom that it pulls the electrons away. This is the scenario where Mr. Lieberman gave all his candy away to Katie.
We also had a pop quiz at the end of class.
Have a great rest of the weekend everyone!
~Kaitlyn Y.
The next scribe will be Elim.
Thursday, March 3, 2011
Build the Big Ones Lab

ethanol

acetic acid

serine

styrene
For each molecule we had to determine the central atom, the geometry of the atom, an example would be tetrahedral, the bond angle for that geometry and the hydridization. Here is what me and my partner Katie I. cam up with:

Lastly, we had to do our conclusion for the lab and if any of you had trouble, this is the conclusion Katie and I came up with:

I hope our data table and conlcusion help! Well that was all we did in period 6 today.
The next scribe is......................................Kaitlyn Y.
Wednesday, March 2, 2011
Continuation on Electron Pairs and Start of Hybridization
Tuesday, March 1, 2011
My. Last. Post. Heck. Yeah. It's on 3D Molecular Design.



Monday, February 28, 2011
Resonance (My Second to last post!!!:))
Keep the jeep rolling people! Now, back to resonance. For an overview, the Wikipedia is a good starter:
In chemistry, resonance or mesomerism [1] is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula. A molecule or ion with such delocalized electrons is represented by several contributing structures
A few of these words may be a bit complicated. Therefor, I will expand upon it in a simpler manner:
Basically, Resonance happens when there are two different bond types when between 3 or more atoms of 2 elements. For example, let's say you have the element NO2-. Well, if you did the Lewis dot structure, you would get this result:

Notice that there is one double bond, and one single bond. Well, for reasons unbeknown to me, this is not how the element really exists. Instead, they exist in a way that is "in between" (Liebs described it as "one and a half" so it's easier to visualize, though it is not technically one and a half). Therefor, this is how they should be drawn:
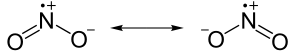
Sunday, February 27, 2011
Covalent Bonding


Thursday, February 24, 2011
Another Exciting Post From The Mind of Ethan Spalding About the Wonderful Topic of: Ionic Bonding
Periodicity (sorry for delay)
Wednesday, February 16, 2011
Battleship and Relations to the Periodic Table
Sunday, February 13, 2011
Electron Configuration
Electron configurations is determined by the sublevel energies and the element. An electron configuration is a short hand for showing where certain electrons are located on a certain atom of an element. Electron configuration is the assignment of quantum numbers to each electron in an atom of a given element. Electron configuration shows the number of electrons (which is the exponent or superscript after the letter) in each sublevel. For instance the book gives the example:
1s22s22p5
Which means that there are two electrons in the sublevel 1s and 2 in the sublevel 2s and 5 electrons in the sublevel 2p. This means that this particular element has 9 electrons which means it is Fluorine.
 When it is written as 1s2 this refers to the electron being in the region of N = 1.Which means that the only "l" option that is possible is 0 (thats zero not "O" like in oh no!) or "s". Because l is 0 there are only two electrons that can be there, and since the 1s sublevel is full (which it must be to begin filling up the next sublevel) the superscript says two (meaning two electrons). If we continued writing a substance's Electron configuration we could go on for a long time but since this has only 9 electrons it would stop at 2p. Each letter ("s", "p", "d", "f") has a top level of electrons of which more than such it cannot hold. for instance, "s" can only hold 2 electrons maximum, "p" can only hold 6 electrons maximum, "d" can only hold 10 electrons maximum, and "f" can only hold 14 electrons maximum.
When it is written as 1s2 this refers to the electron being in the region of N = 1.Which means that the only "l" option that is possible is 0 (thats zero not "O" like in oh no!) or "s". Because l is 0 there are only two electrons that can be there, and since the 1s sublevel is full (which it must be to begin filling up the next sublevel) the superscript says two (meaning two electrons). If we continued writing a substance's Electron configuration we could go on for a long time but since this has only 9 electrons it would stop at 2p. Each letter ("s", "p", "d", "f") has a top level of electrons of which more than such it cannot hold. for instance, "s" can only hold 2 electrons maximum, "p" can only hold 6 electrons maximum, "d" can only hold 10 electrons maximum, and "f" can only hold 14 electrons maximum.
So for example when all are full it would look like this:
1s22s22p63s23p63d104s24p64d104f14
Which has 60 electrons so this indicates that it would be the element Neodymium.
Electron Configuration is a simple way of writing out the electrons positioning of an element, which is important to know for doing other things, and can tell us a lot about an element.
soooo... yeah. thats Electron configuration, Electron configuration, Electron configuration, Electron configuration which makes the tenth time I've said Electron configuration... 11 actually. So thats about it we will learn more on monday, i think, i dont actualy plan the lessons but whatever, the next scribe will be: Joshua D-D-D-EIN!!! (said in monster truck rally voice)
thankyou, and farewell, i will see y'all Monday.
Thursday, February 10, 2011
CHEM IS COOL
A couple of things to take care of:

Wednesday, February 9, 2011
Noting Wednesday
In case you didn't know, we did notes all day today. We learned a lot more about light energy and wavelengths. First, we learned that wavelengths and frequency are indirectly related, so the longer the wavelength, the lower the frequency, and the shorter the wavelength, the higher the frequency. We can use the equation λv=c, where λ is in meters/wave, v is in # of waves/second, and c is the speed of light, which is 3x10^8.
Next, we learned about the electromagnetic spectrum which looks like this:

This gives you a nice visual and adds how long each wave is, so that's helpful. We can only see a very small sliver of the spectrum, specifically the rainbow. This is the area that says "visible" in the picture.
After that, we learned about two very interesting scientists who had opposing ideas about where light came from. Max Planck explained that the transfer of energy was not continuous, and that the energy was quantized, like rungs on a ladder. He believed that light came in waves and believed that it could be explained through his formula ΔE=hv where ΔE is the change in energy, h is Plank's constant 6.626x10^-34, and v is velocity.
On the other hand, Albert Einstein believed that radian was made up of a stream of partciles called photons. He did agree that the energy was quantized, though.
Solving the mystery was Louis de Broglie, who applied the wave-particle theory to electrons. There was a "dual nature of light". His equation is λ=h/mv, where λ is the wavelength, h is Planck's constant, m is the intial mass, and v is velocity.
Remember that the energy in a wavelength is QUANTIZED and has to be a whole number!
During this part of the class, Liebs gave us some really cool psychedlic glasses that allowed us to see the light coming off a helium light bulb. This is something like what we saw when we put them on.


We then went on to learn that when an electron has a very high energy drop, this is what causes it to have a high frequency. Liebs demonstrated this by getting up onto the table and then jumping back down. Also, if an electron has a low energy drop, it has a low frequency. This happens at the speed of light and is impossible to see with the naked eye.
Then we learned something truly astonishing. Niels. Bohr. Was. Wrong.
According to Bohr's model of the atom, the electrons moved in orbits around the nucleus, staying on a similar course the entire time. But, if this was true, a loss of energy would cause the electron to spirial toward the nucleus and crash into it. Obviously, this does not happen, as Ben T. pointed out because otherwise, we would be blowing up all the time.
Instead, electrons move in orbitals. They are different from orbits, as each electron moves around in its own cloud. The atom is mostly empty space except for the nucleus and the regions were you would find an electron. The probability of predicting where the electron is is very possible, but no one can predict it exactly accurately, because they are just regions of space. There is about a 90% probabilty of finding an electron, for it is very vague.

That was about all we did today. I would post the notes, but they aren't on slideshare :( But this is about all we did. Also, the first question on the worksheet we got today is for homework, get it here!
Have a nice Wednesday!
The next scribe will be.........Matteo Parque, enjoy!
:)
Wednesday, February 2, 2011
Entropy and Gibbs free energy
GIBBS FREE ENERGY
Defined: the energy in the system that is available to do useful work.
It is given this symbol and can be calculated like this
Remember that Gibbs free energy is measured in Kj/Mol
THINGS YOU SHOULD KNOW
- If
is negative the forward reaction is spontaneous
- If
is negative then A+b ------> C would be spontaneous
Δ G Reaction Behavior Negative Proceeds spontaneously to the right Zero Is at equilibrium Positive Will not proceed
Below is the entropy post
ENTROPY SHENANIGANS
We started off class today with lab review I am going to type on of the reactions that we reviewed and if you have any questions about the second two reactions, post a comment and I, or someone else, will do their best to answer.
REACTION ONE
Mg + 2HCl ------> MgCl2 + H2
You can use q=mc
BUT WAIT HOW DO YOU GET THE MASS!!!!
Though only the people who got the Mg would know you use a simple conversion factor of
After that you would find the moles of magnesium by these equation
REMEMBER – this was done twice – you need to do the process above two times and then calculate the average. THIS GOES FOR ALL THREE REACTIONS DON’T FORGET THIS THE MORE ACCURATE YOU ARE THE LOWER YOUR PERCENT ERROR WILL BE
Once you have all three of the equations done you need to use Hess’s law AND SHOW YOUR WORK. This work should be shown in the evidence section that lap
CHEM NOTES
Seeing As the slide share link always takes me to gun manuals I am going to type out my notes.
- We started with spontaneous reactions
- A SPONTANEOUS REACTION IS A REACTION THAT TAKES PLACE ON ITS OWN WITHOUT OUTSIDE FORCES
- there are a few things to be worried about though, LIKE
i. It does not have to start on its own so long as it carries out the rest of the experiment
ii. If it spontaneous in one direction then it is not spontaneous in the other direction
- Examples of spontaneous reactions
- Hydrogen ballon reaction
- Paint can explosion
- Ice cube melting
- RUBBER BAND
i. This was the one we spent the most time on
1. The reason that it is such a good example was because when a rubber band is left to sit, it is going to sit there and not move. No matter how much you want something to happen without touching the rubber band, it wont happen. However if stretched out and then contracted, that contraction is a spontaneous reaction because it goes from being stretched out to a normal state with no outside influence
ii. You should not that spontaneous reactions have nothing to do with speed. Below is a great picture for the visual learners out there. if it doesn't show up the it is also here
iii. 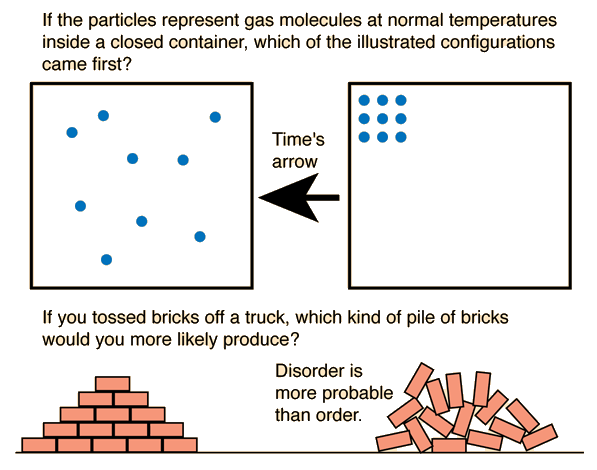
- Nature allows spontaneous reactions
i. Nature divides spontaneous reactions in two main ways
1. Maximum probability – this is where everything mixes
2. Minimum probability – nature has the chance to become disordered but does not
- ENTROPY
- Entropy is denoted with “S”
- Defined as – the increase in disorder or randomness
- REMEMBER TO USE j/mol K NOT Kj/mol K
i. Nature always wants to move toward a positive entropy
- MICROSTATES: different ways molecules can be distributed
i. Increase of microstates = an increase of entropy
ii. large number of microstates = large probability of different states = higher entropy
- FACTORS THAT INFLUENCE ENTROPY
i. Liquid has a great entropy than solid
ii. Gas has the highest
- CALCULATING ENTROPY
i. Very similar to calculating enthalpy
1. It is
2. REMEMBER COEFFICIENTS ARE USED IN THE EXACT SAME WAY WHEN CALCULATING ENTROPY AS THEY ARE WHEN YOU ARE CALCULATING ENTHALPY
a. If there are 2mol of something you multiple by 2 etc
http://www.youtube.com/watch?v=B4SFv_2Skdc&feature=related
This video does a good job summarizing the lesson today.
Alright well I am about done there are just a few announcements you should be aware of
- Today is Kathryn J’s birthday if you haven’t wished her a happy birthday DO IT
- Mr. Lieberman has his ear pierced, though he never wears it
- The lab is due tomorrow
- There is a web assign due Wednesday
- The class would like to congratulate Mollie and Emilio for… Well, being Mollie and Emilio ;)
- We got a Worksheet today that you should do
- Everything for this chapter including both sets of book problems will be due on Thursday and if we have a snow day on Wednesday well then I am not sure.
That’s about it for today. The next scribe is Kathryn J, enjoy



