A peek inside the everyday happenings of our classroom. This is an interactive learning environment for students and parents in my Honors Chemistry 173 class. This ongoing dialogue is as rich as YOU make it. Visit often and post your comments freely.
Monday, December 27, 2010
Monday, December 13, 2010
Bubbles
First, we cut a piece of magnesium in half and measured its length because we would not be able to find the mass on a scale. Magnesium is .01085 g/cm. Our piece of magnesium was 2.5 cm. Then, we tied a piece of copper wire around the magnesium ribbon so that the magnesium would not float around in the eudiometer. (http://www.flickr.com/photos/hc1011/5258241891/) The eudiometer is a gas collection tube, measuring gas by water displacement. We poured 10 ml of HCl and 100 ml of water into the eudiometer. The temperature of the tap water was 21.3°C. Room temperature was 22.2°C and the barometric pressure was 30.11 inHg (we have to convert it to mm). We put the cork attached to the copper wire and magnesium at the open end. We then flipped the eudiometer over, safely and carefully, and put the cork end into a beaker with water and clamped the eudiometer so it would stay up. We watched as the magnesium reacted with the HCl and created bubbles of hydrogen. (http://www.flickr.com/photos/hc1011/5258850050/) Slowly, the water was going into beaker and leaving space at the top of the eudiometer. While we waited for the reaction to finish, we recorded the room temperature and the barometric pressure at room temperature. Once the reaction stopped bubbling, we took the eudiometer to the fume hood and measured the volume of the gas. The volume of the gas was 27.4 ml of hydrogen. (http://www.flickr.com/photos/hc1011/5258850358/)
This lab is due Wednesday. Our test for this unit is Friday. Sorry about the pictures, they won't show up. So I included the links to them.
This has been Kaitlyn. (with a Y!) Thank you, thank you very much. :)
The next scribe will be Matt P!
Friday, December 10, 2010
More About Gas Equations and...Ice Cream
Next we went over our pre-lab. I want to thank Matt P. with some help from Ben A. for explaining to us how to answer each question. Here is how to do them:
1) Make an equation to help find the pressure of hydrogen: 19.8+x=746 and when you solve for x you get 726.2 mmHg of H2.
2)Use your answer from #1 and you will make an equation following the form of PV/T=P2V2/T2. The equation is 726.2(31)/295=760V/273. Solve the equation for the volume of hydrogen at STP and you should get .274 L.
3)This is just a simple stoich problem: .028g Mg x 1 mole Mg/24.3g x 1 mole H2/1 mole Mg =.001167 moles of H2
4) Use your answers from numbers 2 and 3 to get the molar volume of hydrogen at STP: .274/.001167 = 23.5 L/ 1 mole.
Next, after going over the pre-lab, we continued going over notes. The main thing we talked about in our notes is that there are three equations that come from the equation PV=nRT. Those three equations are:
-PV=(g/mm)RT
-mmP/RT=g/v and g/v equals density
-mm=DRT/P~ a way is to remember this is dirty pee...get it..
Also, a reminder, make sure you are keeping up with your worksheets which are due on the day of the test which is on Friday, the day before break.
Then we did an awesome experiment that we were actually able to eat. We made chocolate ice cream! The ingredients were sugar, milk, whipping cream, chocolate syrup, and what made the magic happen, liquid nitrogen. It was and looked a little clumpy, but it actually tasted really good. And that was the end to another good day in chem.
The next scribe will be......Kaitlin Y!
Thursday, December 9, 2010
Gas gas gas
Half of our classmates were gone today...
because of ....... don't know...
Denna wasn't here today so I am the scriber for today
We started the class with a demontration. Mr. Liberman put some melted dry ice into a styrofoam box and put a manometer,this manometer has a empty metal ball, into that box. And the pressure went down because the moles which is in that ball starts to move slow. Through this experiment we could make sure that when temperature goes down then pressure goes down and temperature goes up then pressure goes up.
After Mr. Liberman finish off with this experiment, we wanted him to dump the melted dry ice on the floor.
And he did....Twice :)
The melted dry ice started to move around the floor like when we spill some water on the hot pan.
We were all excited about the movement of these little particles.
Also Mr. Liberman dumped the ice over my head accidently!
Korri screamed and called 911 immediately
So that's why I am in hospital right now...
hehe I'm just kidding~
Actually he poured vapor over my head. It just feel coooool~
Today, we learned about the Ideal Gas Equation.
The three important gas laws, Boyle's, Charles', and Avogadro's, derived relationships between two physical properties of a gas, while keeping other properties constant.
When we rearrange to a more familiar form we get PV = nRT
Wednesday, December 8, 2010
What it really Boyles down to
Then we went on to discuss conversion between temperatures of celcius and kelvin because it is necessary to know for formulas. The reason you need to know the Kelvin temperature is because if you use celcius you may end up with correct calculations stating that you ave negative density, which i hope all of you know is "impossible" according to Mr. Lieberman. To convert celcius to Kelvin all you need to do is add 273 degrees to the celcius measurement.
Then as we continued our notes we went on to Charles' law, which states: The volume of each gas is directly proportional to temperature. V=bT where b is a constant and V1/T1=V2/T2. This means that the original volume over temperature will be equal to the new volume over temperature. To explain this to us he showed us a quick experiment by heating to small flasks with water until they boiled, he then stuck a balloon on top of one which was still boiling and the balloon filled as the gas expanded. he stuck another balloon on top of the other right after removing it fro the heater, the balloon was quickly sucked into the flask because of the rapid change in temperature and the fast reduction of volume.

Next D Liebs quickly explained Avogadro's law which states: Volume is directly proportional to the number of moles of gas. To show this he simply blew up a balloon and let go, letting it soar around and land on Alex's desk. This demonstrates that as moles of air are released there is less and less volume and pressure to keep the balloon inflated, so it eventually collapses. then there is the infamous combined gas law which is P1V1/T1=P2V2/T2 this can be used to apply all forces to one equation instead of several different ones.
There was also a sheet to pick up at the front when we walked in, that sheet is homework and is due come test day. Speaking of which is next friday, the friday right before break. Also, everyone check out the little map thing to the right, people all over the world have seen our blog!
Tuesday, December 7, 2010
The pressure is on
http://www.flickr.com/photos/hc1011/5241587429/
We also learned the different units for measuring pressure, and the conversions between them. They are: pounds per square inch (psi), atmosphere (atm), Torr, or millimeters of mercury (mmHg), and pascal (Pa). The conversions are: 14.7 psi=1 atm=760 mmHg=100 kPa. We also went over how barometers and manometers work, with the atmosphere pushing down to show the pressure.
We went over Dalton's law too, which states that the sum of partial pressures of gases equals the total pressure of the gases when combined. So, if gas 1 is 1 atm and gas 2 is 2 atm, when they are combined the pressure is 3 atm. Boyle's law deals with pressure and volume, stating that the product of the pressure and volume for a gas equals a constant, k (PV=k). So no matter what differences in pressure or volume occur, P1V1=P2V2.
We also got to see Brandon and Zoe get stuffed in a trash bag and have the air sucked out to demonstrate the actual air pressure here on earth, as well as see an inflated balloon expand when the air is sucked out. Happy late arrival tomorrow, and the next scribe is Peter W.
http://www.flickr.com/photos/hc1011/5241588237/
http://www.flickr.com/photos/hc1011/5241588451/
ChemThink gases
Tuesday, November 30, 2010
Percent Yield
Sunday, November 28, 2010
Pumpkin Pie Reactants
On Tuesday we turned in our Copper and Silver Nitrate Labs, and our first set of problems in the "HomeFun Problem Set." Mr. Lieberman went over 2 of the homework problems, #67 and #68, before we turned the assignment in. We further reviewed over limiting reactants during class.
Over the break make sure you do the Copper Cycle pre-lab.
If you need a copy of the lab here is the link: Copper Cycle
To help you get in the limiting reactants thanksgiving mood, here are the actually ingredients to make a pumpkin pie:
 1/3 c. firmly packed brown sugar
1/3 c. firmly packed brown sugar1/2 c. sugar
1 tsp ground cinnamon
1 tsp ground ginger
1/4 tsp. ground cloves
1/2 tsp. salt
1/2 tsp. ground nutmeg
2 eggs
1 1/2 c. evaporated milk
2 c. pumpkin puree
You have the following ingredients available:
3 c. firmly packed brown sugar
4 c. sugar
5 tsp ground cinnamon
5 tsp ground ginger
6 tsp. ground cloves
6 tsp. salt
6 tsp. ground nutmeg
6 eggs
3 c. evaporated milk
6 c. pumpkin puree
a. How many pumpkin pies can you make?
b. Which ingredient limits the number of cakes you can make?
Monday, November 22, 2010
To find out which one is the limiting reactant, there are two ways.
The first way, you could find out how much of one reactant you need to react with the other. We used Al for this one. So we take the mass, .82g Al, multiply it by 1 mole over it's molar mass, then find the mole ratio of Al and CuCl(2) (which is 2 to 3), then multiply it by the molar mass of CuCl(2). Since the answer (6.1g CuCl(2) was more than what we have (.87g CuCl(2)), we can conclude that CuCl(2) is our limiting reactant. If out answer turned out to be less than .87g, Al would be our limiting reactant.
The other method is to find out how much of each reactant you need to produce one product. It's always good to look ahead since this question 99.2% of the time will be right after the first: how much of a product you could make. You would follow all of the above steps for each of the reactants and you would choose a product. We used Cu as our finishing product. Remember, whichever reactant produces the least of a product is the limiting reactant. In the end, CuCl(2) produced the least Cu, which turned out to be .41g. Since that's the most that CuCl(2) can produce, that is all that will be produced. You don't do anything with that number!
So after we react this all, how much Al is left? well... to find that there are two ways you could do it, but both are pretty much the same.
First you could take how much of the limiting reactant you have and find out how much of the excess reactant you need to react it, which then you subtract that from how much you start with. To get this, just follow the steps listed in paragraph 2, except for the limiting reactant.
The other way is to take that .41g Cu and see how much of that excess reactant (Al) you need to react with that. It's basically all the same as listed above with different variables.
After we got through all of that, we got to our worksheet, Limiting Reactants, which is part of our homework.
For tomorrow, We need to have our Lab which we recently did and our chemistry question set #1.
1 silver troy ounce = $27.46, I got part of your homework done.
If you're still reading, please come up to me tomorrow and give me a waffle.
We will most likely have a quiz tomorrow, we were lucky not to have one today.
The next scribe will be... Austin W.
Sunday, November 21, 2010
Beginning of the End
Thursday, November 18, 2010
How to Make Silver for Pinkie Toe Rings
Tuesday, November 16, 2010
Pop Goes the Weasel!
2C2H2 + 5O2-> 4CO2 + 2H2O
From this Mr. L asked us how many grams of H2O will form if .60 grams of CAC2 reacts. This is the formula we got:
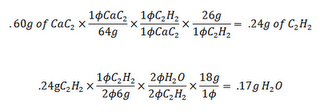
We ended up with .17 grams of water. Now it was time to put this formula to the test. Mr. Lieberman loaded up his mole-rocket with the special ingredients from the formula. It was the combination of the CaC2 and H2O that created the Acetylene gas. This gas was the ignition that the rocket needed to make the mole fly. Suddenly there was a BOOM! A little red object shot across the room followed closely by a trail of fire. There were a few screams, particularly from Korri, which was expected. The room then filled with a smell that was immediately placeable: It was a mix of burnt hair and/or that smell from straightening your hair when it is still slightly damp.
Next, we all took our 4 question quiz. It was then graded and handed back. Hope everyone did well! And the next scribe issssss........Mollie M!
Monday, November 15, 2010
BOOM!
Tuesday, November 9, 2010
Insolubility and Solubility
To make things more interesting, Mr. Lieberman did the Lead Iodide experiment to show that certain compounds are always soluble (NO3), and therefore, you are able to identify the solids in a reaction. See Video Below:
Monday, November 8, 2010
Lots of Reactions

In class today, we started a lab with many different sodium and nitrate groups, totaling 48 reactions. Why would we do that many, you ask? Well it has to be either:
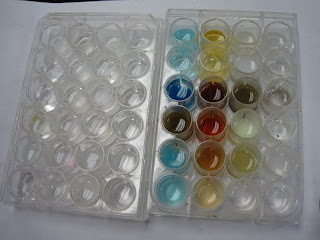 And my data table, if you can read it:
And my data table, if you can read it: And the post lab is the real fun part: we have to write chemical equations for EVERY SINGLE PRECIPITATE! Both molecular and net ionic. For example, the molecular equation would be: 3Na2SO3+2Al(NO3)3 yields Al2(CO3)3+6NaNO3 and the net ionic equation would be: 2Al3++ 3CO32 yields Al2(CO3)3. And repeat. About 20 times. Just a thrilling homework assignment. Oh, and by the way, for those who can't realize it yet: NaNO3 is a product for every single molecular reaction, and always dissolves.
And the post lab is the real fun part: we have to write chemical equations for EVERY SINGLE PRECIPITATE! Both molecular and net ionic. For example, the molecular equation would be: 3Na2SO3+2Al(NO3)3 yields Al2(CO3)3+6NaNO3 and the net ionic equation would be: 2Al3++ 3CO32 yields Al2(CO3)3. And repeat. About 20 times. Just a thrilling homework assignment. Oh, and by the way, for those who can't realize it yet: NaNO3 is a product for every single molecular reaction, and always dissolves.Thank you for reading my post. I hope you all do wonderful on your labs now. Now, the next scribe will be:.............................................
Sunday, November 7, 2010
Keeping Balancing Equations Cool Like They're Going Out of Style
- Molecular Equations show the overall stoichiometry of the equation, but not the actual forms of the substances
- (Example): KCl+AgNO3------------>KNO3+AgCl
Complete Ionic Equations
- Complete Ionic Equations represent, as ions, all substances that are strong electrolytes
- Ionic Compounds dissociate in water
- Spectator Ions are ions that don't react; spectator ions are still included in Complete Ionic Equations
- (Example): K(^+)+ Cl(^-)+NO3(^-)------------>AgCl+K(^+)+NO3(^-)
Net Ionic Equations
- Includes only those ions undergoing a charge (spectator ions are NOT included)
- Net Ionic Equations are only written if a solid is formed
- (Example): Cl(^-)+Ag(^+)------------->AgCl(s)
NOTE: Molecular Equations, Complete Ionic Equations, and Net Ionic Equations are all Double Replacement Reactions
After we deciphered Molecular Equations, Complete Ionic Equations, and Net Ionic Equations, we proceeded to work on our newest worksheet (Net Ionic Equation Worksheet). Here are some tips in completing this worksheet:
- To find the Molecular Equation, balance the equation given
- To find the Complete Ionic Equation, seperate ions from compounds (include charges)
- If a reaction isn't seen, or there is no solid, a Net Ionic Equation isn't needed
We proceeded to work on the Net Ionic Equation Worksheet for the rest of class.
On Monday, we will be doing a series of 48 reactions. The reactions will help establish rules regarding what we talked about on Friday. Furthermore, there is a WebAssign due Monday, on section 4.2 (Precipitation Reactions).
Stay thursty, my friends.
Thursday, November 4, 2010
The Scream Heard 'Round the World
 Today, class started off with Mr. Lieberman telling everyone to get out their Classifying Chemical Reactions Lab so we could go over the post-lab questions, which asked you to find the equations for some experiments that you did during the lab. People wrote the answers on the board and we cleared up any questions about them. For answers see Brandon L's previous post that described the lab's experiments and their equations.
Today, class started off with Mr. Lieberman telling everyone to get out their Classifying Chemical Reactions Lab so we could go over the post-lab questions, which asked you to find the equations for some experiments that you did during the lab. People wrote the answers on the board and we cleared up any questions about them. For answers see Brandon L's previous post that described the lab's experiments and their equations. Wednesday, November 3, 2010
Can You Classify Chemical Reactions because I hope I can
Today and yesterday in Lieb's class we started off by talking about the 3 worksheets we need print off of moodle after this we began our class notes on the 5 types of reactions: Synthesis, Decomposition, Single Replacement, Double Replacement, and Combustion Reactions.









Monday, November 1, 2010
Intro to Balancing Equations
two very important rules to remember, and that Mr. Lieberman was very persistant in reminding us was:
1. DO NOT CHANGE THE SUBSCRIPTS
2. There are no stead fast rules to balancing equations, so the best way to master this skill is to practice, practice, practice.
After showing us these liberating notes he demonstrated a couple experiments for us. Both of which come with an entertaining story.
1. The Water from hydrogen and oxygen eperiment
It all started when Mr. Lieberman was taking a strol in deserted mountains with no one around and no water. Why he was walking in the heat without water or people in general is a mystary to us all. Well, while he was on this hike he got thirsty, but without any water and no connection to civilization he was stuck in the middle of nowhere without a source of water. This caused a problem for Mr. Lieberman, but it just so happened that a hydrogen spring was there and he was carrying two rulers, a balloon and a match. Attaching the balloon, which he had filled up with hidrogen from the spring, to one of the rulers he was ready to make water. His idea was that if he caused a reaction to occur he could make H2O out of the hydrogen and the oxygen in the air. He ran the experiment and low and behold, there was water (or at least some form of it). He demonstrated this awesome experiment in our class:
Along with this riviting story, came the story of his pet elephant Stampy:
Now Mr. Lieberman owns a pet elephant and this elephant is known as Stampy. Stapmy has needs just like any other elephant, and this includes brushing his teeth. Mr. Lieberman being the thoughtful man that he is makes his own elephant toothpaste, just for his Stampy. He demonstrated the making of this special toothpaste for us in class:
These stories made the class interesting and educational, depicting scinerios that use the topics we are learning of in class. Don't forget to write up the prelab including any tables needed for the procadure. If you don't do that you will get no credit for the WHOLE prelab, as I have had to learn from experience.
And now your next scribe will b.......Brandon L. Congrats
Sunday, October 31, 2010
Friday's Class
this type of mole:
We used this on the test and many people did very well. After answering all of the quetions about the test, we got time to go over to the computer lab and work on the ChemThink that is due on Monday. Make sure you finish that if you have not done so already. The next scribe will be....Mollie M!!!
Tuesday, October 26, 2010
Magnesium Oxide Lab
At the beginning of class, Mr Lieberman checked in our pre-labs (don't forget that means a data table as well) and we got started on the Magnesium Oxide Lab. We began by massing an empty crucible and lid, and then massing the crucible, lid, and a 25 cm magnesium ribbon together. We recorded these two values in our data tables and then proceeded to place the covered crucible on the clay triangle. We lit the burner and removed the lid every three minutes for a total of fifteen minutes. After the flame had been carefully turned off and the crucible cooled down, we massed the crucible, crucible lid, and magnesium oxide product.
For the post lab:
1. we are first asked to calculate the mass of the magnesium metal and the mass of the product, using the law of conservation of mass to calculate the mass of oxygen that combined with the magnesium. (Law of conservation mass: the mass of substances in a closed system will remain constant, no matter what processes are acting inside the system). This should be done by subtracting the mass of crucible, lid, and ribbon from the mass of the crucible, lid, and magnesium oxide.
2.Then, we are asked to calculate the percent composition of magnesium oxide. To do this, start by calculating the formula mass, and dividing each component mass by the formula mass and multiply by 100.
3. We are then asked to use molar mass to calculate the number of moles for each reactant. (see class notes)
4. calculate the ration between the number of moles of magnesium to the number of moles of oxygen and find the empirical formula of magnesium oxide. Look at Elim's post if you still need help with empirical formulas!
5. convert mass to moles to find theoretical yield (this shouldn't be too complicated)
6. calculate your percent yeild
Hope this helps! Tonight's homework is to complete Avagadro's Crash Activity. If you still need help on empirical formulas I found the following video very helpful http://www.youtube.com/watch?v=r2Log6-voWo! Also, work on the lab (due Thursday), and don't forget to begin studying for Thursday's test! Tomorrow's scribe is Katie I!
Monday, October 25, 2010
Empirical vs Molecular Formula
Then we started to learn what empirical formula is, and how this Empirical formula relates with Molecular formula.
Once you get Empirical formula, then easy to get Molecular formula.
The Empirical formula is the simplest form of the Molecular formula.
For example :
Molecular formula - C6H12
Empirical formula - Divide by 6 - CH2
Here is a sample we did in the class:
Determine the Empirical and Molecular formulas for a compound that gives the following %.First, assume a 100g of the sample
- 71.65% (Cl)
- 24.27% (C)
- 4.07% (H)
The molar mass is known to be 98.96g/moles
- 71.65g (Cl)
- 24.27g (C)
- 4.07g (H)
Second, Convert gram to moles
- 71.65g x 1moles / 35.5g = 2.018 moles of Cl
- 24.27g x 1moles / 12g = 2.02 moles of C
- 4.07g x 1moles / 1g = 4.07 moles of H
Third, divide each moles by smallest number of moles
- Cl : 2.018 moles / 2.018 moles = 1 mole
- C : 2.02 moles / 2.018 moles = 1 mole
- H : 4.07 moles / 2.018 moles = 2 moles
Finally we can get the Empirical formula like this : CClH2
Now, we can find the molecular formula by finding the mass of the Empirical formula and setting up a ratio:
CClH2 → C2Cl2H4
*If you want more information about Empirical formula
I recommend you to visit http://www.chem.tamu.edu/class/majors/tutorialnotefiles/empirical.htm
After we had finished the note, Mr. Liberman introduced an activity. This activity is called Crash of Avogadro Airlines Flight 1023.
The goal of this activity is finding a murderer.
On the first and second side of the sheet, there are 3 tables which help to find the horrible murderer.
In this activity each chemical can be used once or no time.
Tonight's homework is to finish the hydrate lab, do a Magnesium Oxide pre-lab and Avogadro Airline activity which is due on Wednesday.
Just a reminder, the unit 3 test is on Thursday.
The next scribe is Ellen Hirsch
Sunday, October 24, 2010
Hydrate Lab Day!
a. The mass of the hydrate
b.the mass of the water lost during heating
c. the percent water in the hydrate
We were given the mass of:
Empty Test Tube: 18.42 grams
Test Tube and Hydrate (before heating): 20.75 grams
Test Tube and Anhydrous Salt (after heating): 20.41 grams
To calculate these you had to:
a. 20.75 g -18.42 g= 2.33 g
b. 20.75g- 20.41 g =.34g
c. .34g/2.33g=14.59% (round for significant figures)=15%
After this question you had to complete the table given on the Hydrate Lab sheet.After this, we got together with our lab groups and began the Hydrate Lab here are the steps:
- We took the mass of the pyrex test tube.
- We added around 2grams of blue hydrated (with water) copper (II) sulfate.
- We then massed the test tube with the copper (II) sulfate in it.
- We then set up the clamp and tilted the tube slightly down so the evaporating water can leave the test tube.
- Then then focused the heat from the Bunsen burner on the copper (II) sulfate and heated it evenly until it turned white. We tried to avoid burning it. (turned brown if burned)
- Then we wiped out the excess water droplets and massed the tube with the anhydrous salt.
So that was our Hydrate Lab! The next scribe is... Ellen Hirsch!!
This was Korri Hershenhouse! Don't forget to do your How Much Are You Worth Project for monday! Also don't forget to complete the webassign and do the post lab also for monday!
Thursday, October 21, 2010
Who's Worth More: Your Lil Brother or Lil Wayne?
Wednesday, October 20, 2010
'MOLE'ten Lava

Honors chemistry period six is now on round two for these scribe posts because we had to skip Elim.
We, then, moved on to the lab, "% Composition of Bubble Gum," which we did not do a pre-lab for. Mr. Lieberman told us to fit all of the lab on one page, because it is a relatively short one. The procedure was as follows:
- Take the gum and mass it with the wrapper (record)
- Unwrap and chew gum
- During the chewing process, mass the wrapper (record)
- Place chewed gum on wrapper and mass it (record)
- Calculate the mass of sugar in the bubble.
- Calculate the % composition of the sugar (by mass of the bubble gum).
- Calculate the moles of the sugar and gum using the molar masses given the procedure.
- Using your answer from question number 3, determine a possible empirical formula for the bubble gum.
- Researchers have found that the ideal formula for the gum is GS2, where G is a fictional elemental symbol for gum and S for sugar. How does your gum compare to the ideal? What might be some sources of error?
- Assume you have 100 g of the substance (makes the math easier because everything is a straight percent).
- Consider the amounts you are given as being in units of grams.
- Convert the grams to moles for each element.
- Find the smallest whole number ratio of moles for each element.
Tuesday, October 19, 2010
Insert Mole Title Here
Now for class, we had kind of a work day in store for us. We got more practice with moles so we could better understand what it is and also we got more practice with unit conversion.
First off, we went to our notes and finished two "Learning Check!". First problem we had was:
Question: How many atoms of K are present in 78.4 g of K?
Work: Take the amount (78.4 g), multiply it by (1 mole of K over 39 (molar mass) grams of K), then multiply that by (Avogadro's number (6.02 times 10^23) over 1 mole of K).
Answer: 1.21 times 10^24.
Our second Learning Check! was:
Question: What is the mass (in grams) of 1.20 X 10^24 molecules of glucose (C6H12O6)?
So this question is actually working the opposite way of our other problem. We've got to find the mass (instead of molecules) and we start with the molecules (instead of the mass).
Work: 1.20 X 10^24 times (1 mole of glucose divided by Avogadro's number) times (the Molar mass divided by 1 mole of glucose).
To find the molar mass for this problem, we must take all the subscripts, multiply them by the mass of their individual atomic masses, then add all of those together. In this problem, the molar mass would be:
6 (subscript of Carbon) X 12 (atomic mass of Carbon) + 12 (hydrogen) X 1 + 6(oxygen) X 16 = 180 grams
Once we plug in the molar mass, our answer should be 359 grams per mole.
So, in order to really understand how we got this, you must already know about moles and molar mass. If you do not understand these fully, please either look back at your notes (you can find extras in moodle), or just look below at Kaitlyn's post.
After we finished those two problems, we had the rest of the period to work on our mole workshop. This compromised of 5 questions all of which we should have done by tomorrow with our partner. An extra copy of this worksheet can also be found in moodle under unit three worksheets (unfortunately the answers are not there).
Homework: Finish the Mole Workshop as well as the second mole worksheet. Both were given out before class.
Important dates: 10/20 (tomorrow!) review quiz on unit conversion and moles.
10/28 Unit exam, remember to have your homework problems done
Random fact of the day: Black whales are born white.
Also, I'd like to point out we had our first visitor from Japan.
Next Scribe will be Elim J

